4.2.1] Exposures.
In level 2 we present more details about the factors in the three sets in the Venn diagram.
4.2.2] Environmental and lifestyle factors.
4.2.3] Where you live could have an effect on your general health, but unless certain conditions prevail the normal environment in which you live is only going to play a small part in whether you develop cancer.
4.2.4] Solar radiation:
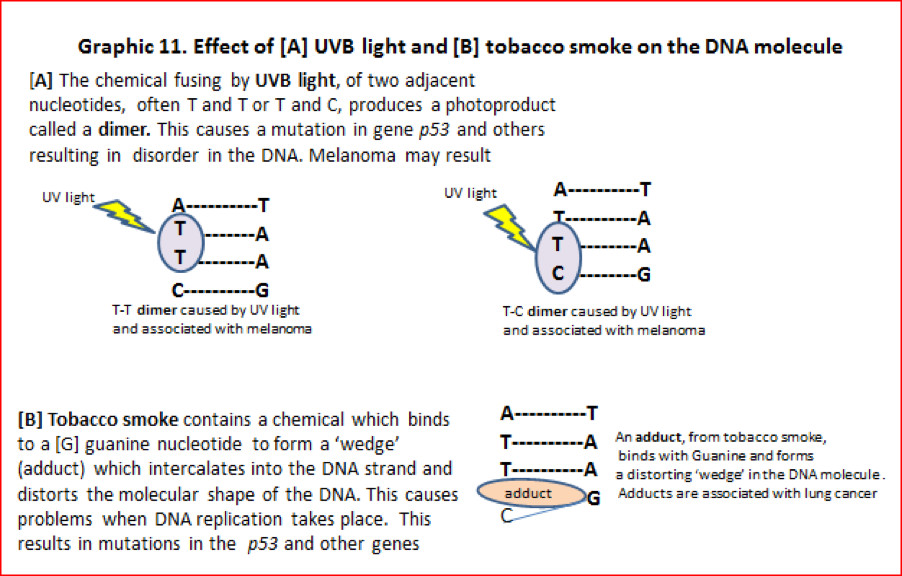
We mentioned earlier that solar radiation is the main cause of skin cancer. The pigment melanin in dark skin offers some protection, but if you are fair skinned exposure to UVB radiation from the sun and some sunbeds, can cause mutations. The UVB light energy causes two adjacent nucleotides of the DNA molecule to join together to form a dimer (See graphic 11[A]). When replication is attempted during cell division the dimer causes the DNA code to be misread often resulting in C changed [→] to T nucleotide transitions. This results in mutations of the p53 and other genes. A build-up of this type of mutation can cause melanoma. Cosmic rays also fall on us from space but on earth they are insignificant in terms of causing cancer. For astronauts this becomes a considerable problem.
4.2.5] Produced ionising radiation: X-rays and fallout from nuclear bombs, nuclear plant accidents (e.g. Chernobyl) and accidental or otherwise leaks from radioactive sources damages DNA. At certain levels this type of radiation can produce double strand breaks (see graphic 12) in the DNA molecule. Double strand breaks are difficult for the cell to repair.
4.2.6] Radon gas: This gas is produced when the radioactive decay of uranium 239 takes place in underground rocks. It can seep upwards into buildings constructed above the rocks. Some lung cancers are associated with radon gas but in the worst affected areas building regulations require that buildings are specially ventilated below floor level.
Public Health England has information and excellent maps about radon distribution.
Graphic 11. Effect of [A] UVB light and [B] tobacco smoke on the DNA molecule
4.2.7] Mutagenic chemicals in smoke from smoking tobacco and exposure to ‘second-hand smoke’ are well-known causes of cancer, especially some types of lung cancer. Problems can start when a chemical in tobacco smoke binds with part of the DNA to produce an adduct structure. (See graphic 11[B]) This distorts the DNA molecule and causes mutations in the p53 and other genes. G→T transitions are found in mutations in the p53 gene in cases of lung cancer victims who have smoked. Smoke from open solid fuel fires used for cooking can also generate mutagenic chemicals. This is a cause of lung cancer in some countries.
4.2.8] Mutations caused through the action of certain viruses and other biological issues.
4.2.9] Viruses: Specific viruses are linked to certain cancers. How this works is not fully understood but it is thought that in some way the virus ‘hijacks’ the cell control system and keeps cell division switched ‘on’ to produce more cells for the virus to populate. An example of a virus linked to cancer is the Human Papilloma Virus (HPV). 13 strains of this virus are highly associated with causing cancer of the cervix, vulva, vagina, mouth, anus and penis. Fortunately a vaccine against HPV is available and is now offered to young women and perhaps, soon, to young men. Both hepatitis B and hepatitis C virus are associated with liver cancer and Epstein-Barr virus is associated with a cancer of white blood cells called Burkitt’s lymphoma. [See also: section 10 ‘Reducing the risk’].
4.2.10] Bacteria, parasites, moulds and the microenvironment: bacteria, parasites and moulds do not cause cancer directly but can give rise to inflammation and carcinogenic or mutagenic chemicals that initiate cancer development. Bacteria that are foreign to humans can also alter the microenvironment around cells. This is generally done by causing inflammation which switches on a ‘cell replication kit’ to repair the damage. An inflammatory site presents a good ecological niche which is conducive to cancer cells developing, and where they can keep dividing. Stomach cancer is an example of this. Helicobacter pylori bacteria create the inflamed microenvironment.
4.2.11] Food, mutagens and carcinogens. The preservatives in some foodstuffs can contribute to the formation of some types of cancer. This is mainly through chemicals in food reacting with chemicals in the gut, and their effect on specific genes.
Sodium nitrite for example is included in processed meats to preserve them. Unfortunately sodium nitrite reacts with acids in the stomach to form carcinogenic chemicals called nitrosamines.
Eating too much red meat increases the level of haem protein containing iron in the body. In people with a deleted APC gene, an increase in haem level switches on a specific (wnt) signalling pathway and this results in uncontrolled cell division.
Charred food such as that produced on a smoky barbeque, can also produce mutagenic chemicals.
4.2.12] Contaminated food.Some fungal moulds that produce carcinogenic/mutagenic chemicals can grow on foodstuffs especially nuts and grain that has been poorly harvested and badly stored. An example of this is the chemical aflatoxin which binds to DNA and prevents normal replication. Aflatoxin is produced by a mould growing on peanuts.
4.2.13] Your work/workplace. The workplace can contain mutagenic and carcinogenic substances and factors that are associated with cancer development. Mutagenic substances and factors can cause mutations (alterations in the DNA code). Carcinogenic substances and factors are also associated with cancer, but do not directly cause mutations. Carcinogenic substances may, for example, cause constant inflammation. In time this could induce cancer formation or cause chemical changes and cancer by an indirect route.
In many countries many carcinogenic and mutagenic chemicals have been banned from the workplace or restricted for use below a ‘threshold limit’.
But not all situations are avoidable. Outdoor workers are often exposed to the sun, a mutagenic factor. Shift work that disturbs the human circadian rhythm may be associated with, but is not yet a proven cause of, breast cancer in women and is classed as a group 2A carcinogen.
4.2.14] Personal lifestyle and life-course events. Your lifestyle can expose you to sources having a ‘mutation potential’ and include: exposure of unprotected skin to bright sunlight, some artificial tanning devices and smoking tobacco. Inhaling ‘second-hand smoke’ and drinking more than the safe level of alcohol (bearing in mind that from a cancer point of view there is no ‘safe’ level). Another contributing factor is eating a lot of red and/or processed meat and charred food cooked on smoky barbeques (smoke is the critical factor in barbecuing).
Obesity through weight gain, especially at waist level, has its dangers. Excess calories support cell proliferation and this is associated with an increased risk of breast, prostate and colon cancer. In post-menopausal women excess fat, working with aromatase enzymes, probably synthesises the hormone oestrogen which is associated with cell proliferation and some types of cancer.
High birth weight is associated with an increased risk of all childhood cancers. It is thought this is due to the higher number of cells increasing the ‘mutation potential’. All this sounds rather depressing BUT most of these factors ARE controllable.
(See also section 10: Reducing the Risk)
Life-course events: some of these are under your control but others have personal and societal influences in the form of demands and constraints. An example of this is whether a woman bears a child/children and whether or not she chooses to breast feed the child. As a result of the hormonal differences produced, women who do not bear children have a slightly higher risk of breast cancer.
4.2.15] Societal influences
The human race is ‘on’ and social evolution is running against biological evolution……….. and the winner is……probably cancer?
This statement illustrates what Professor Greaves calls ‘Evolutionary Mismatch’ – a mismatch between evolutionary adaptations and our modern lifestyles.
Humans have an exceptionally high risk, about 1 in 2 in the UK, of developing a clinically defined cancer and at a sub-clinical level we probably all develop tumours during our life. Why does society suggest that it is acceptable for fair skinned people to burn their non-pigmented skin in the sun to make it look slightly caramel colour, to eat and drink alcohol to excess, and/or to smoke tobacco when we all know the problems that they can bring? [At the time of writing, June 2017, there are reports that, in the UK, less alcohol is being consumed and fewer cigarettes smoked, so perhaps as a society we are learning.]
These, along with many other aspects of cancer, are comprehensively and clearly described in ‘Cancer, The Evolutionary Legacy’ by Professor Mel Greaves. Publ. p/b Oxford University Press, 2000 [Very readable, recommended. The book has been translated into several languages].
4.2.16] Endogenous processes
In previous notes we have seen how some environmental and lifestyle factors are associated with cancer. These factors become internalised at a molecular level within the cell and are joined by mutagenic factors that arise there.
These mutagenic factors include:
4.2.17] Internal errors made during cell division: When a cell divides there is an enormous potential for errors to happen and it has been estimated that at each cell division about 120,000 mistakes (mainly copying errors) are made. This may sound a lot, but the human genome is composed of about 3 billion nucleotides, so relatively speaking the error rate is low.
Fortunately many of these errors are small and detected by the cell’s own quality control system. Repairs can be made provided the repair system is not itself corrupted by mutations (see later). If beyond repair, the cell is recycled through the apoptosis, programmed cell death, process. (See: BSCB softCell: Programmed cell death).
4.2.18] Physical damage to genes/chromosomes during cell division can produce chromosomal instability. This can occur when centromeres and kinetochores malfunction causing poor, sometimes unequal, segregation of chromosomes. In turn this may produce: too many chromosomes, too few chromosomes or re-arranged chromosomes e.g. as in aneuploidy (see note 3.2.22), translocation [see note 3.2.21 and graphic 6 in section 3 and chromothripsis (see note 3.2.23 and graphic 4(b)]).
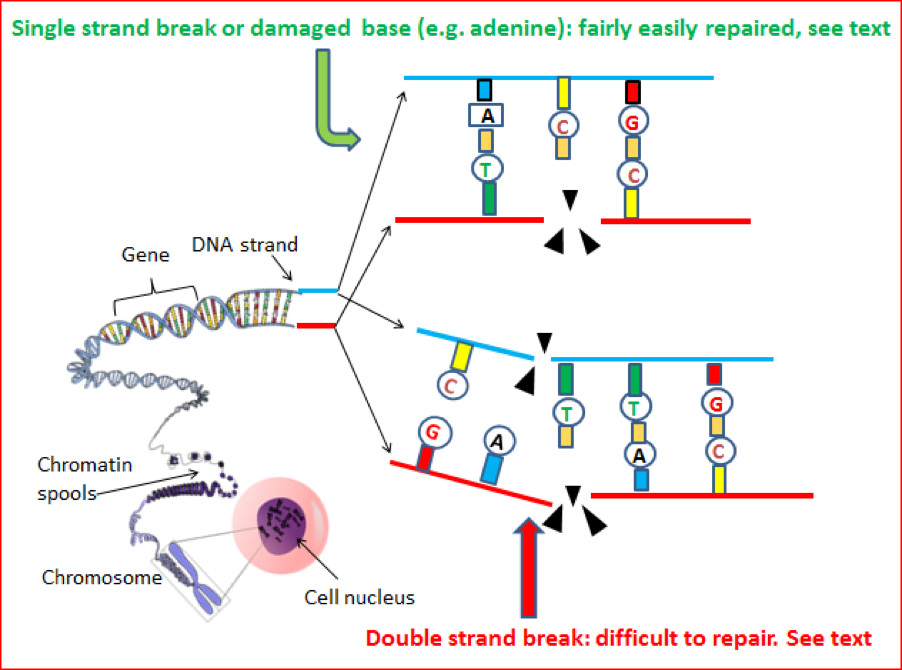
4.2.19] Breaks in the strands of the DNA molecule. These include double strand breaks (DSBs) which are really bad news for a cell, and single strand breaks (SSBs) which can more easily be repaired.
Graphic 12: Physical and/or chemical damage to DNA strands.
Single strand damage accounts for most of the errors occurring in DNA. Fortunately damaged nucleotides (A,T,G,C) and physical breaks in the ‘backbone’ of the strand can be repaired fairly easily. Double strand breaks, although fewer in number, are a greater problem. Sometimes the damage is so bad the cell is shut down by apoptosis. Double strand breaks are associated with chromosome fragmentation [see graphic 4(b) in section3] and translocation [see graphic 6 in section 3].
4.2.20] Other endogenous (internal) factors include: (1) damage to genomic DNA due to exposure of DNA to chemical stresses brought about by the generation of free radicals of oxygen and nitrogen and the oxidation of DNA, (2) Mismatching of bases, ‘A,T,C and G’, (3) the persistent production of cell proliferation hormones. (4) the production of chronic inflammatory conditions and (5) the effect of epigenetic and chromatin regulation factors.[see: BSCB softCELL: Epigenetics] The effect of these two points in factor (5) is the subject of current research but in both cases it is thought that the factors may play a part in regulating cell activities including replication and repair aspects in cancer progression.
4.2.21] Suppression of the immune system. If the immune system is compromised in any way, cancerous cells stand a better chance of survival. The converse of this is that a healthy immune system has been shown to help suppress some cancers. The whole field is the subject of intensive research.
4.2.22] Inherited genetics
People can remember and note through their family tree, relatives who have suffered from cancer. Whether they might inherit a particular ‘family cancer’ will be of concern to them.
The number of major cancers that are heritable (also called familial or germline) is relatively small but with some the ‘penetration’ is high. This means that if the mutated gene(s) are inherited there is a high risk, >70% chance of the cancer developing. This is why some people have preventable, proactive surgery to minimise their perceived risk of developing a familial cancer. A much publicised case of such action was that taken by actress Angelina Jolie.
It is important to note that whilst some people will develop cancer due to a familial predisposition, many more will develop the same type of cancer through a non-familial or somatic route.
4.2.23] The four most common inheritable cancers are: cancers of breast, ovary, prostate and bowel. There are several other quite rare but familial cancers, but the Li Fraumeni syndrome (LFS) and Lynch syndrome (LS), are of special interest because they are associated with the potential of developing many types of cancers.
4.2.24] Li Fraumeni syndrome (LFS). This is a rare familial disorder but with a high penetration in affected families. The cause is a particular mutation in the p53 gene [the ‘guardian of the genome’ gene]. The normal gene and its products are important for the smooth running of the cell cycle. It is not surprising therefore that it’s mutated form is associated with leukaemias, brain tumours, breast cancer, lymphoma, adrenocortical cancer and many more cancers.
4.2.25] Lynch syndrome (LS). This is a type of bowel cancer (hereditary non-polyposis colorectal cancer [HNPCC]). It does not cause symptoms of its own, but people with Lynch syndrome have a higher risk of womb, ovarian and other bowel cancers and it is also associated with cancers of the stomach, pancreas, small intestine and ureters. Lynch syndrome is generally caused by mutations in ‘mismatch repair’ genes.
4.2.26] Breast cancer: an example of a cancer that can have familial or somatic origins. A predisposition to breast cancer can be produced by both somatic and germline mutations. One in eight women in the UK will develop breast cancer during their life. (Data: CRUK and NHS choices). A rather smaller number of men will also develop breast cancer.
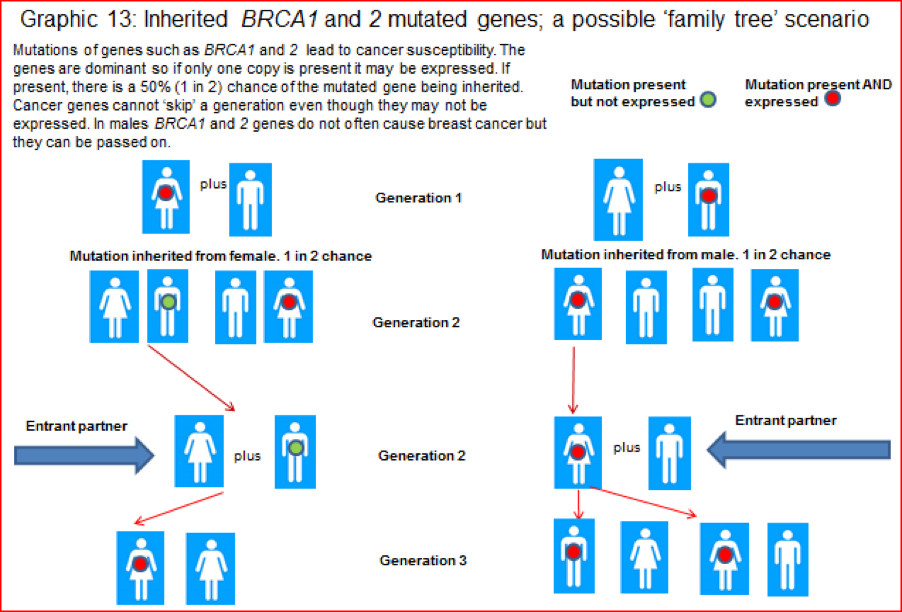
While mutated BRCA1 and 2 genes are definitely linked to a predisposition to breast cancer, breast cancer can be caused by mutated but rarer genes including CHEK2, PALB2, RAD51B, RAD51C, PTEN and not so rare p53.
4.2.27] BRCA 1 and BRCA 2: the so called ‘breast cancer genes.’ These two genes are probably the most well-known genes. We all have them, and in their normal non-mutated state they are tumour suppressor genes and play a very important role in the repair of DNA and cell damage and promote the normal growth of breast, ovary and other cells.
Undoubtedly having one or both BRCA genes mutated can have a profound effect not only because of creating a predisposition to breast cancer, but also due to creating, in women, a predisposition to ovarian cancer, and in men a predisposition to prostate cancer.
4.2.28] What causes harmful mutations to BRCA genes? It is known that the BRCA 1 gene resides on chromosome 17 and BRCA 2 gene on chromosome 13, but what causes the mutations in every case is not clear. Fragmentation of chromosomes (as in chromothripsis – see Section 3, graphic 4(b) and chromosome translocations (see Section 3, graphic 6) can play a big part. At a molecular level breaking of DNA strands is probably the basic cause.
4.2.29] Chance.

Chance to possibility
Changes by chance are happening to the DNA in our genes all the time. It has been calculated that the average mutation rate is between 10-8 and 10-9 per base pair per cell division cycle. New cells, especially in the bone marrow and intestinal lining, are being produced at the rate of 1011 cell per day. Couple these facts and the result is that within your body ‘zillions’ of mutations happen every day. Fortunately most of the mutations will not cause problems BUT there is a CHANCE that a mutation or mutations will produce a cancerous cell or cells. This means that there is a POSSIBILITY that a clinically significant cancer could form. This has led to the idea that during a human life-course everyone develops covert cancer.
4.2.30] Possibility to Probability (likelihood). Whether a cancer cell progresses from being a possibility to a greater likelihood of cancer developing, depends on many factors. Some of these will be considered in more detail in Section 6, but In general terms the likelihood increases when elements in the three sets in the Venn diagram are present (the red intersect area in graphic 10). The likelihood also increases as an organism increases in age. This is because, over time, more potentially harmful mutations can accumulate, and, the body’s natural restraints on cancerous outgrowths are probably less effective.
4.2.31] The chances of inheriting faulty genes in breast cancer. Graphic 12 shows a possible simple inheritance pattern for BRCA genes, but, as mentioned before, cancer is complicated. The level of probability of having a predisposition to breast cancer depends on how close a relative you are to someone with an identified BRCA gene mutation and other factors.
Breast Cancer Now (a UK charity) has two helpful tables that indicate the chances of inheriting a faulty gene. They can be found under the section headed ‘Family history of breast cancer. Managing your risk‘.
End of section 4.