3.2.1] What causes cancer?
In most cases cancer is caused by certain mutations (or changes) in the DNA ‘instruction manual’ which is located in the nucleus of the cell. Mutations can be the ‘big changers’. We will look at them next.
3.2.2] What are mutations?
In a mutation the order of the ‘letters’ of the DNA ‘alphabet’ are changed. This takes place in a section of the DNA sequence that codes for a specific function. Such a section is called a gene.
The human genome contains over 3 billion nucleotides (the DNA ‘letters’ A,T,C,G). Humans have about 20,000 genes and this makes up about 1-2% of the genome. Genes are distributed amongst the chromosomes and it is the genes that code for proteins which then produce specific proteins that signal instructions to cells.
3.2.3] Mutations are normal biological events
Mutations are a major part of Darwinian style evolution and much of biological evolution is driven by mutations. Without mutations there would be no diversity for natural selection to work on and we would not have evolved into our present state of development.
Many mutations are ‘neutral’ or ‘passenger’ and confer neither advantage nor disadvantage. But, other mutations can be deleterious and can modify DNA in such a way that genes that include the modified DNA may direct the making of the wrong protein and protein products, or the correct protein in the wrong quantity in the wrong place at the wrong time; or no proteins at all.
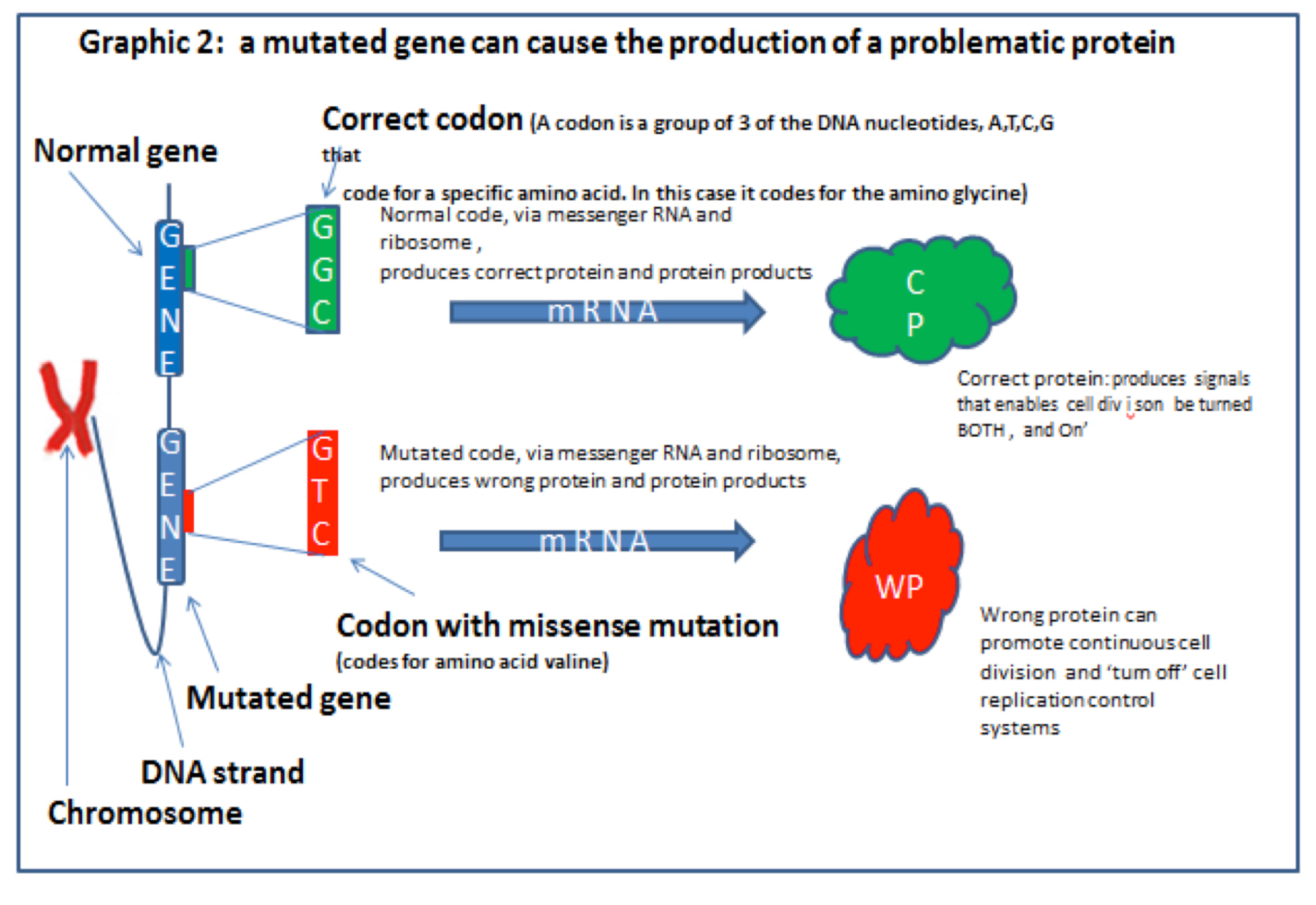 Most genes in their normal form are part of ordered and healthy metabolism. It is only when they are mutated in certain ways that they create disorder and sometimes cancers.
Most genes in their normal form are part of ordered and healthy metabolism. It is only when they are mutated in certain ways that they create disorder and sometimes cancers.
Cancer is not alone in being caused by corrupted DNA code. Corrupted code also gives rise to several disorders including cystic fibrosis, Huntington’s chorea, and sickle cell anaemia. Mutations are brought about and influenced by various factors both internal and external to the cell [see section 4, what causes mutations?]
3.2.4] There are two groups of mutations: germline and somatic
3.2.5] Germline mutations are inherited from our parents and may bring with them a predisposition to certain disorders including some cancers. Some types of prostate and breast cancer are examples. This does NOT mean cancer will definitely develop, but that the chances of it doing so are increased. As in genetics generally, some mutations are dominant and some are recessive
3.2.6] Somatic mutations are not inherited and are sometimes called ‘sporadic’ and can occur by chance (sometimes called ‘bad luck’) or through the influence of other factors including epigenetic and environmental ones.
3.2.7] Genes…what’s in a name? (BRCA 1, BRCA2, p53, RAS and MYC)
[Convention: gene names printed in italic; protein products of genes are NOT in italic]
In the general media you may well hear about ‘the gene for a particular cancer’. For breast cancer the BRCA 1 and BRCA 2 genes are well-known examples, but this does not convey biological detail. All of us, male and female, have BRCA genes and other so called ‘cancer genes’; they are part of our normal genome. BRCA normal genes for example are involved in the extremely important process of the day-to-day repairing of damaged DNA which then helps maintain the stability of the cell’s genetic material.
Perhaps we should not say “the BRCA gene for breast cancer” but “the BRCA mutated gene for breast cancer”?
3.2.8] Tumour suppressor genes (TSGs)
Genes of this group have a normal function of keeping a controlling and restraining role on cell division. They are able to slow down cell division much as a brake does on a cycle or car. Mutated TSGs lose their braking effect. With no restraints on cell replication cells divide out of control.
In normal gene expression there is something of a ‘steady state’ or homeostasis at work between tumour suppressors and proto-oncogenes. Problems start when the two become unbalanced.
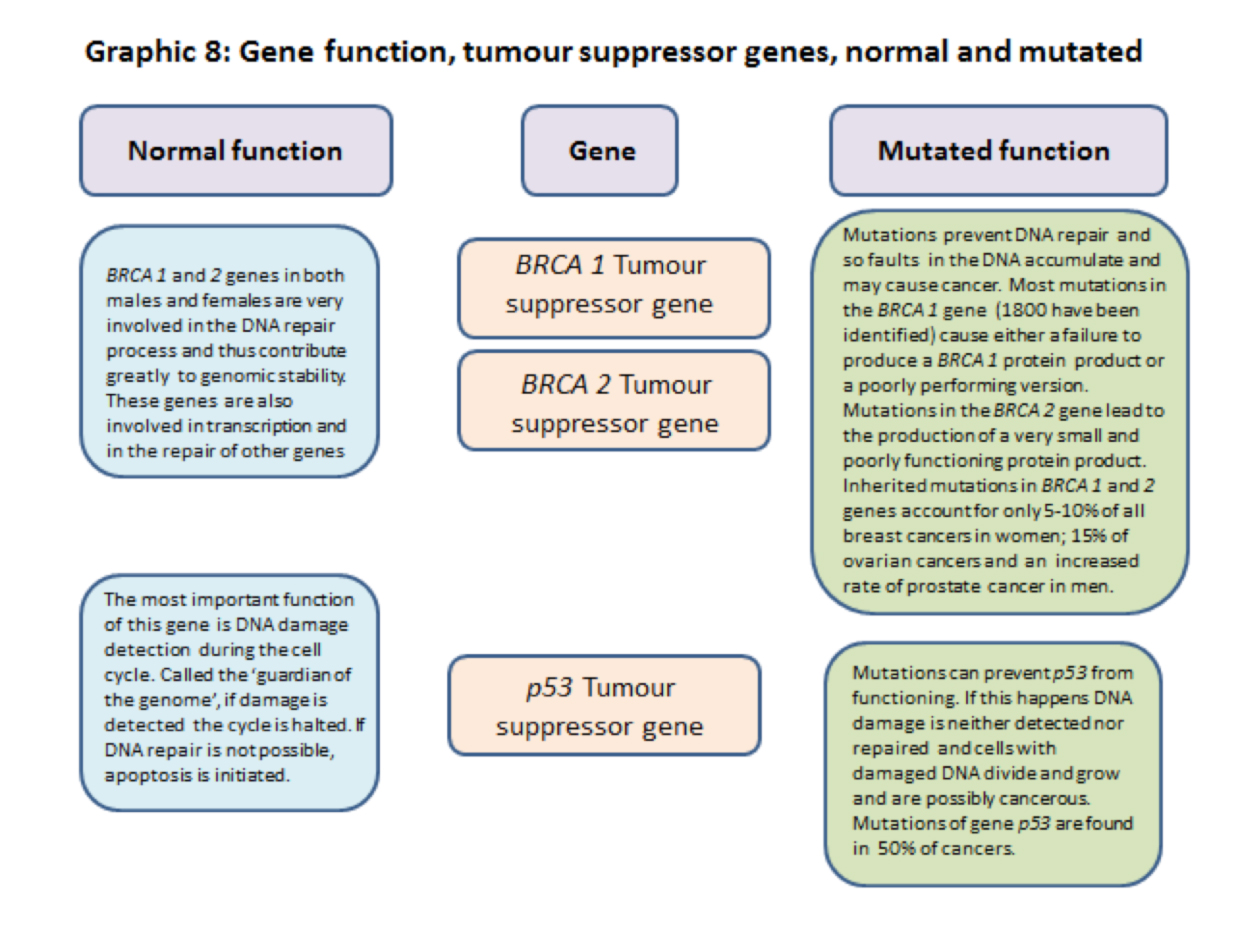
3.2.9] BRCA1 and 2 are tumour suppressor genes.
In addition to controlling and restraining functions BRCA genes are very involved, with other genes and proteins, in the process of DNA repair and hence genome stability.
They are also involved in the transcription of DNA code into RNA code. When BRCA genes are mutated DNA damage repairs are not done and cell division races ahead unrestrained and many cells with damaged DNA are created. These are potentially cancerous cells.
3.2.10] p53 tumour suppressor gene. – The ‘Neighbourhood Watch’ gene that becomes the ‘Guardian of the Genome’ gene.
The p53 gene and its protein products are well known, perhaps even famously, in cell cycle and cancer biology, but are not attached to a particular cancer by name. This is probably because over 70% of human cancers have mutations that interfere with the working of p53. Sometimes the gene is deleted completely but mutations affecting its normal working have been recorded in over 6,000 mutations.
During normal surveillance activity p53 ‘lies low’, it’s ‘Neighbourhood Watch’ mode, when it keeps a constant ‘look out’. In its role as a ‘guardian of the genome’ and of the cell cycle process in particular, its main function is to work as a ‘quality control inspector’.
If p53 detects damage the level of p53 rises considerably (more ‘quality control inspectors’ are brought in) and the cell cycle is slowed down. This is to enable repairs to be made to the damaged DNA. If the damage is too great to be repaired reasonably and safely, (just like a damaged car) p53 will scrap the cell by causing the cell mitochondria to trigger cell death by apoptosis.
If the p53 gene is mutated it exhibits loss of function; its surveillance ability is negated and damaged DNA will be replicated producing rogue cells in quantity, all with damaged DNA. These rogue cells may well contribute to cancer development.
Several viruses, including human papillomavirus (associated with cervical cancer), have evolved ways of inactivating p53.
3.2.11] Proto-oncogenes and oncogenes
Proto-oncogenes are a group of genes whose normal function is to promote cell division and sometimes inhibit cell death. In normal cell metabolism proto-oncogenes work with other genes and signalling pathways. Normally their activity is kept in check by regulating and restraining cell signalling messages including some from tumour suppressor genes.
Proto-oncogenes are changed to oncogenes by mutations which bring about an increase in gene activity leading to an increase in the level of chemicals that promote cell division. In many cases this happens through gene duplication or chromosome translocation and the production of a fusion chromosome (such as the Philadelphia chromosome) through which a high level of cell division promoting chemicals are produced.
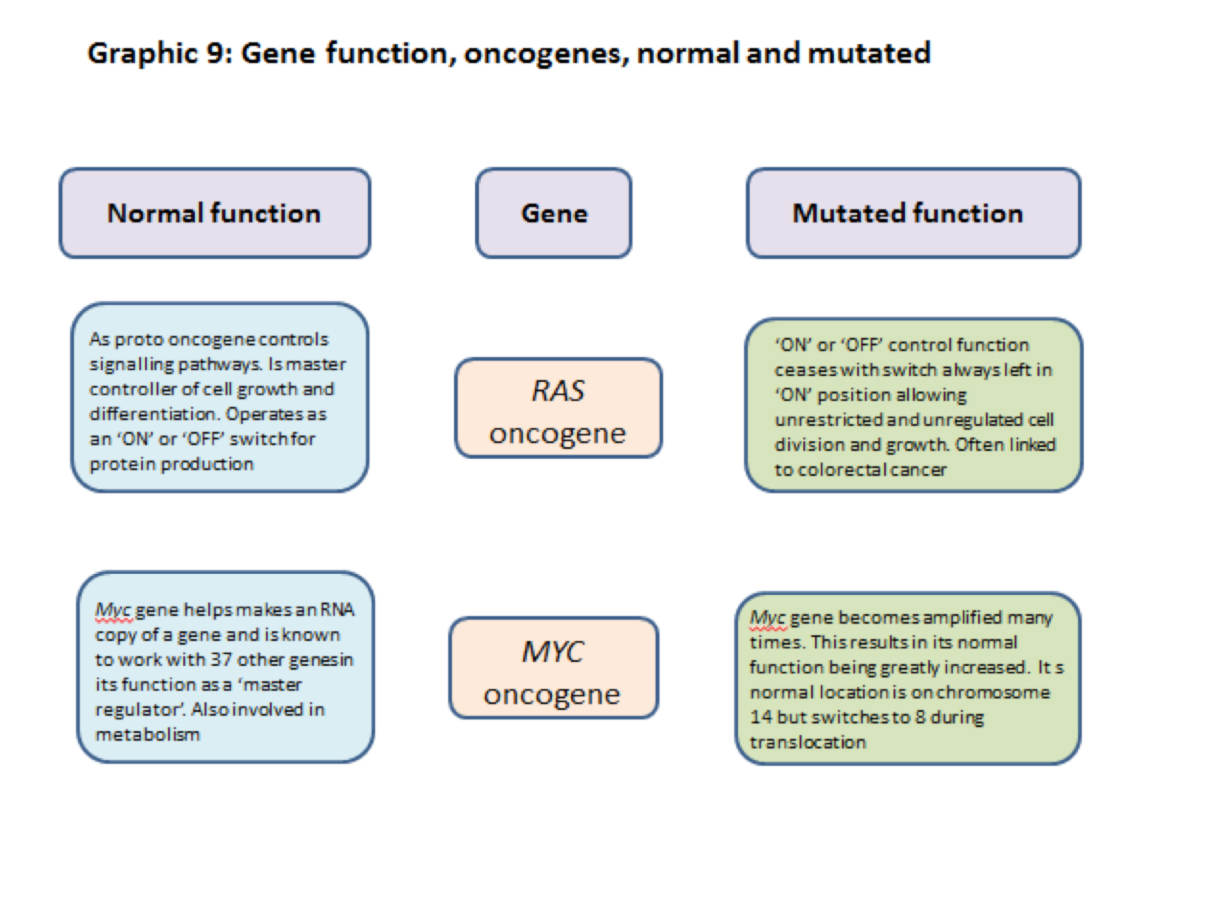
3.2.12] RAS proto-oncogene/oncogene
The RAS normal gene is a proto- oncogene and in its normal state promotes cell division and growth. It is responsible for signalling the production of protein products that turn cell division ‘ON’ and ‘OFF’ i.e. it is a controller of cell division.
The RAS mutated gene keeps the signal ‘ON’ all the time and promotes uncontrolled cell division and growth and cancer development.
 When mutated the function of RAS is amplified and it promotes uncontrolled cell division and growth.
When mutated the function of RAS is amplified and it promotes uncontrolled cell division and growth.
3.2.13] MYC proto-oncogene/oncogene
The Myc normal gene is a ‘master regulator’ working as a transcription factor with at least 37 other genes in regulating the production of proteins. In many cases the proteins produced are involved in increasing the rate of cell division.
When mutated the Myc gene goes into ‘overdrive’ producing elevated levels of cell- division promoting chemicals.
The Myc gene is normally located on chromosome 8 but is sometimes translocated to chromosome 14 where its capacity to promote cell division is greatly amplified. This can cause problems. (see 3.18[3])
3.2.14] Not all mutations are equal
There are several types of event that can create a mutation. Some of them have a greater potential than others to cause problems.
Events are of two types: small scale and large scale.
3.2.15] Point mutations, (small scale events):
Point mutations may have a big impact. They are the most numerous type of mutation and frequently found associated with cancer. It is important however to remember that a single mutation in one cell MAY NOT cause a problem; [just as leaving out the letter ‘u’ from the word ‘color’ does not affect the meaning].
The same mutation happening many times in one cell, [see duplication, below] or in many cells, could produce a problem as when skin cells are over-exposed to sunlight.
Whatever the type of event they ALL involve changes in the DNA code and how it is ‘read’.
When the code is read it has to be read in blocks of three ‘DNA letters’ (called a CODON), this is because each block of three nucleotides (the ‘letters’) of a DNA strand codes for one (but occasionally two), amino acids or the instruction ‘STOP’. Graphic 3 (below) from Professor Nazneen’s blog explains this.
 3.2.16] Macromutations, (large scale events):
3.2.16] Macromutations, (large scale events):
Macromutations are often associated with cancer and there are six main types of these mutations. The ‘big six’ are:
- Duplication of Genes. When a particular gene is duplicated so is the protein product.
- Inversions Parts of chromosomes become turned upside down
- Missing genes. Genes can go missing during cell division
- Translocations. Parts of chromosomes break off and translocate to and fuse with other chromosomes
- Aneuploidy. This is when there are more, or less, chromosomes present than there are in the normal cell nucleus.
- Chromosome shattering. Call chromothripsis this is when many chromosomes fracture and the resulting parts may fuse with one another in a chaotic way.
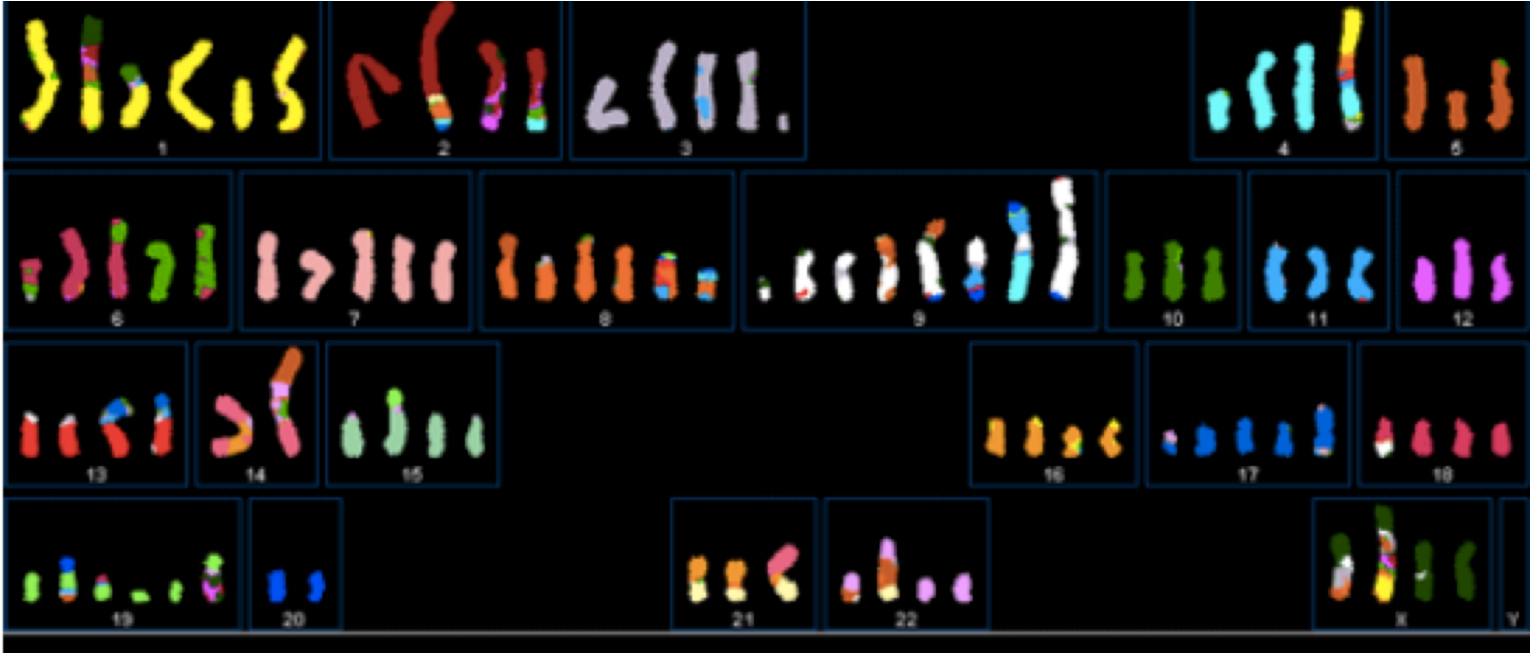
In some types of breast cancer chromosome fracture, fragmentation and fusion can be extensive.
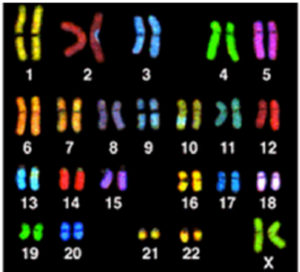
Graphic 4(a) This image shows ‘painted chromosomes’ (called a spectral karyotype [SKY]) of a normal human cell.
Graphic 4(b) This SKY image shows fragmented chromosomes from a particular type of breast cancer cell. NOT ALL BREAST CANCER CELLS SHOW THIS DEGREE OF FRAGMENTATION. Image courtesy: Drs P Edwards and M Grigorova at http://www.pawefish.path.cam.ac.uk/BreastCellLineDescriptions/HCC1954.html
BUT, whatever the type of event disordering of DNA is at the heart of the problem.
3.2.17] Macromutations, (large scale events):
The following macromutations are often associated with cancer:
3.2.18] Duplication of genes. As the name suggests a gene can be duplicated one or more times along the chromosome. Gene duplication may cause the abnormal over-production of a protein product. If this product promotes cell division directly or indirectly, cancer may be an issue. Duplication of the Myc gene is associated with colorectal, gastric, breast, uterine and pancreatic cancers.
Gene duplication: an example with a short code: The following is the normal but duplicated, ‘DNA code’ for the gene for the peptide leu-enkaphalin (an opium type chemical found in animals)
stop TAT GGG GGG TTT CTC stop TAT GGG GGG TTT CTC stop. This gene duplication would probably cause the over-production of this opioid. Result unknown!
3.2.19] Inversions: these happen when a section of a chromosome breaks off and is then reinstated but in an inverted position. This alters the ‘reading’ of the DNA code.
 3.2.20] Missing genes. Complete genes or parts of them can go missing as a result of errors during mitosis. Depending on the extent of the missing part, a different protein may be produced, or no protein at all. Using the gene in [1] above as an example, a partly missing gene might read as: stop TAT GGG … … CTC stop (lost codons of gene: GGG TTT).
3.2.20] Missing genes. Complete genes or parts of them can go missing as a result of errors during mitosis. Depending on the extent of the missing part, a different protein may be produced, or no protein at all. Using the gene in [1] above as an example, a partly missing gene might read as: stop TAT GGG … … CTC stop (lost codons of gene: GGG TTT).
3.2.21] Translocations. In this type of event one or more chromosomes physically break across their diameter and the resulting parts fuse onto other chromosomes or parts of them. Evidence of translocations are found in many cancer cells.
This happens in chronic myeloid leukaemia (CML) where parts of chromosome 9 translocate to chromosome 22. The resulting fusion forms a chimeric fusion chromosome and in this particular case is called the ‘Philadelphia chromosome’ after the name of the laboratory town in which this translocation was discovered.
The Myc gene is often associated with translocations between chromosomes 8 and 14 with the Myc gene moving from chromosome 8 to 14. Here it is able to greatly amplify the production of cell division promoting chemicals. Part of chromosome 14 translocates to chromosome 8 in the ‘switch’, see graphic 6 below.
 3.2.22] Aneuploidy: sometimes there are more, or less, chromosomes present than there are in the normal cell nucleus. This is due problems during cell division and a result of chromosome breakage. Aneuploidy is quite common in some types of cancer.
3.2.22] Aneuploidy: sometimes there are more, or less, chromosomes present than there are in the normal cell nucleus. This is due problems during cell division and a result of chromosome breakage. Aneuploidy is quite common in some types of cancer.
[Chromosomal rearrangements in which extra chromosomes are produced are the cause of disorders such as Down syndrome in which 3 copies of chromosome 21 are made.]
3.2.23] Chromosome shattering. Called chromothripsis, this is when many chromosomes break and when the resulting chromosome parts can fuse with other broken parts in a chaotic way. Fortunately this is not common and clearly the outcome is highly variable.
3.2.24] Some mutations in more detail
We will now look at a point mutation and a macromutation in more detail.
3.2.25] Point mutation. The gene chosen is the RAS gene [name derived from RAt Sarcoma] gene of the RAS gene family. In this example it is mutated by a missense mutation (see graphic 3, above). The mutated RAS gene family has been found in about 95% of mutations in tumours in pancreatic, colorectal, lung and ovarian cancers and in about 1/5th of melanomas.
In its normal state the RAS gene is involved in very important (kinase) signalling systems. It is through these signalling systems that the RAS gene takes part in the promotion and control of cell growth and differentiation. This is done by the gene signalling to proteins to produce products that promote cell division, cell growth and survival. The RAS normal gene controls this process by switching a promotion switch chemical ‘ON’ or ‘OFF’ as required, to maintain cell production at the correct level.
When the RAS gene is mutated it can no longer signal the production of the protein product that can turn the promotion switch ‘OFF’. As a consequence the promotion switch stays ‘ON’ and unregulated cell division proceeds towards forming a tumour. Of course, being biology things are rather more complicated than this, but basically this is what happens. The next stage of oncogenesis (the development of cancer) involves a process that is as old as life itself! See section 6
Before the next example take a break and see whether you can spot a missense mutation in the following sequences of DNA data within a RAS gene.
[3.2.26] Mutation spotting: are you a good DNA sequence analyser?
Can you spot the missense mutation in part of the DNA sequence of the RAS mutated gene in row 2 compared with the same section of the RAS normal gene in row 1?
Raw data arrives from machine sequencing in this form: …….ATGACGGAATATAAGCTG….
Part of the DNA sequence of a RAS normal gene divided into codons to make it easier for you to read:
[Row 1]
ATG ACG GAA TAT AAG CTG GTC GTG GTG GGC GCC GGC GGT GTG GGC AAG AGT GCG GTA ACC ATC CAG CTG ATC CA………
Part of the DNA sequence of a RAS gene with a missense mutation. Can you find it?
[Row 2]
ATG ACG GAA TAT AAG CTG GTC GTG GTG GGC GCC GTC GGT GTG GGC AAG AGT GCG GTA ACC ATC CAG CTG ATC CA………..
(idea and data courtesy of Dr Robin Hesketh from his book ‘Betrayed by Nature’.
Publ h/b Palgrave Macmillan 2012 [Very readable good information, recommended])
How did you get on? It looks easy but it is not, especially if you are distracted!
The mutation you probably spotted is just one of those that can occur in the RAS family of genes that could initiate cancer. It usually takes more than one mutation, but mutations can accumulate over time.
If searching sequences appeals to you, you can see more examples of KRAS and BRAF gene sequences on the website: www.yourgenome.org the schools/public website of the Wellcome Sanger Institute, [Cambridge, UK]. Quite a lot of modern biology includes looking for patterns in data and sometime making predictions from them.
3.2.27] Macromutation. To illustrate a macromutation we are going to use a translocation which is responsible for Chronic Myeloid Leukaemia (CML). As cancers go the biology of CML is fairly straightforward, but in this respect it is unlike other cancers which are much more complicated
The translocation involves parts of chromosomes 9 and 22. In the normal state the abl gene of chromosome 9 regulates, through protein products, the transfer of phosphate from adenosine triphosphate (ATP) to another protein which controls the production and growth of white blood cells. In effect the abl gene regulates whether white blood cells are produced or not.
CML can be initiated when a biological accident happens during mitosis. Chromosomes 9 and 22 each sustain one break across their diameter. Parts of each chromosome then exchange places with each other and fuse with the other chromosome. Part of chromosome 9 fuses with part of 22, and part of 22 fuses with chromosome 9. See graphic 7, below.
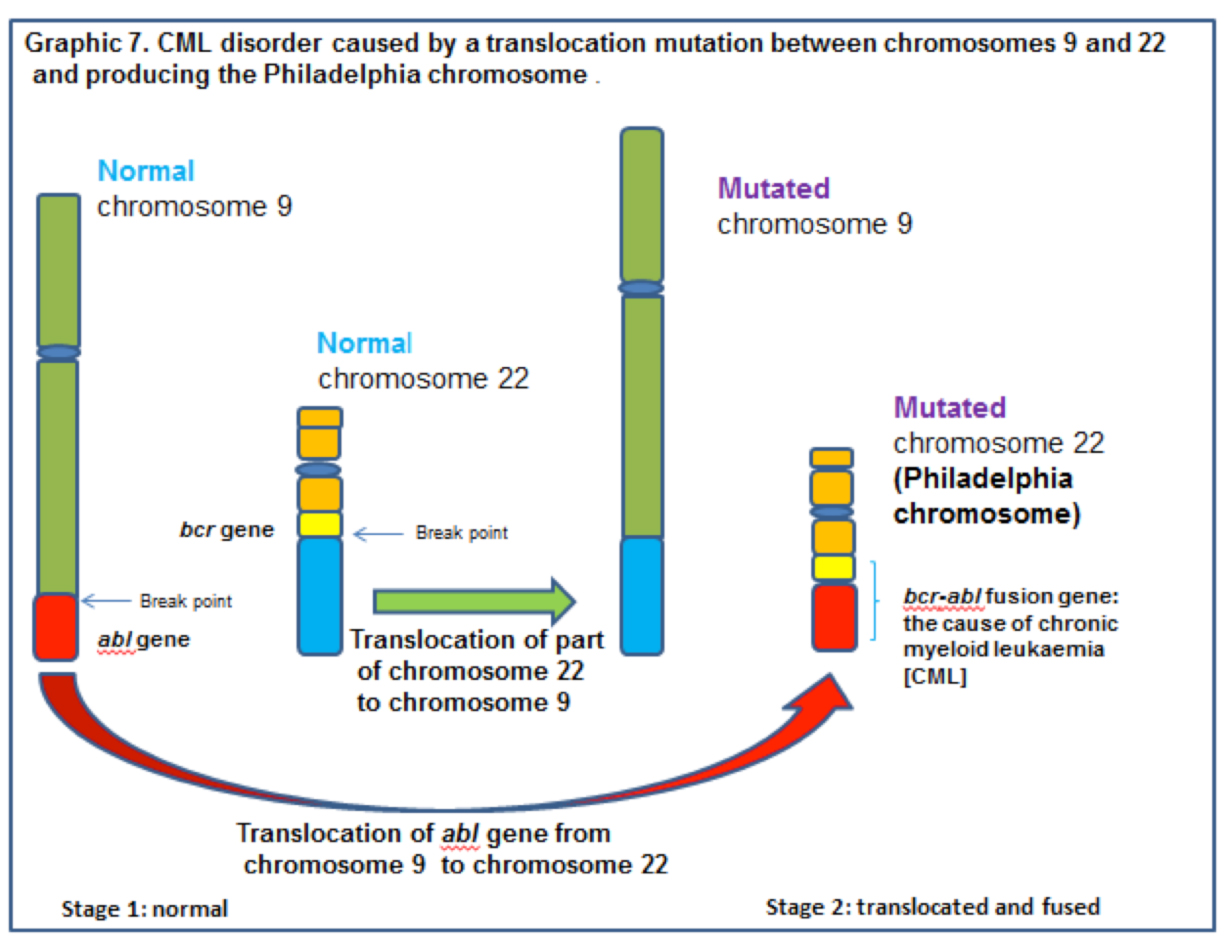 This translocation results in the abl gene from chromosome 9 fusing with the bcr gene on the now shorter than normal chromosome 22. Unfortunately the newly formed bcr/abl fusion gene is an oncogene and the protein product (tyrosine kinase) of this gene is a promotor of cell division and white blood cell replication. Fused to bcr, the abl gene cannot be turned ‘OFF’ and white blood cell production does not stop.
This translocation results in the abl gene from chromosome 9 fusing with the bcr gene on the now shorter than normal chromosome 22. Unfortunately the newly formed bcr/abl fusion gene is an oncogene and the protein product (tyrosine kinase) of this gene is a promotor of cell division and white blood cell replication. Fused to bcr, the abl gene cannot be turned ‘OFF’ and white blood cell production does not stop.
The now short chromosome 22 is called the Philadelphia chromosome after the town in which the laboratory work was conducted. [CML can now be controlled by the use of a tyrosine kinase inhibitor called imatinib mesylate (Gleevec). Unfortunately, like may cancers, the cells evolve to a position in which Gleevec becomes ineffective.]
A summary of some of the functions of ras, myc, brca1, brca 2 and p53 normal and mutated genes are shown in graphics 8 and 9 below.